Conductivity
Many fast reactions do not result in measurable changes in absorbance or fluorescence. A large number, however, do exhibit a change in conductivity as the reaction progresses. Examples include organic and inorganic oxidation and reductions, proton exchange, metal-ligand complex formation, surfactant aggregation to form micelles, and ion-exchange.
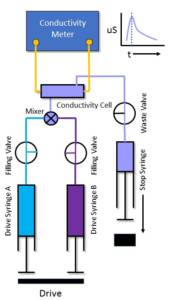
The use of a stopped-flow system with the capability to perform conductivity measurements can, therefore, considerably extend the scope of this already powerful technique.
Conductivity measurements offer greater signal-to-noise and a much better dynamic range than spectrophotometric measurements. For example, a five volt signal can be produced from conductance ranging from 500 000 µS to 5 µS, depending on the amplifier range setting and the noise on each of these is only 0.01%. Reactions are generally characterised before the kinetic study is carried out to ensure that conductivity changes are due solely to the reaction of interest.
Conductivity measurements can be made in the presence of a large, but constant background signal so it is possible to work with comparatively large buffer concentrations and to use non-aqueous ionising solvents such as ethanol and acetone.
TgK Scientific are able to provide a dedicated conductivity instrument (CSA-20) or as is more widely used, a conductivity accessory or dedicated set-up for the KinetAsyst SF-61SX2 Stopped-Flow instrument (OPT-642).
- AN.006.S60
Conductimetric monitoring of proton loss from acetylacetone in excess dilute alkali
Keywords
- Conductivity
- Stopped-flow
- conductimetric monitoring
References
- ¹J. E. Crooks. Hosny El-Daly, Mohammed Y El-Sheikh, Abdul-Fattah M Habib and Ahmed B Zaki, Int. J. Chemical Kinetics, 25, 1993, 161-168.

