Temperature-Jump
The T-Jump technique provides a means to follow fast reactions with half-lives of just a few microseconds. The reaction volume is prepared so that it is in equilibrium and then rapidly perturbed by a rapid change in temperature. There is a new equilibrium constant at the higher temperature, but the initial concentrations are balanced for the lower temperature. The system therefore relaxes and the reaction proceeds until the concentrations have reached their new equilibrium values.
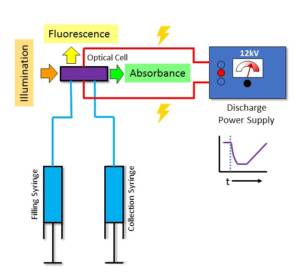
This heating effect is due to a short pulse of electrical discharge at high voltage capable of inducing a temperature rise of up to 10°C within a few microseconds. This method is also referred to as Joule Heating.
The reaction is followed spectrophotometrically by UV/Vis (absorbance and fluorescence) using fast response detectors and signal processing. Thus very fast processes can be characterised by changes in the optical signal.
We have extensive experience in the design and development of T-Jump equipment (TJ-64) for a huge range of chemical and biological systems.
Typical applications include fast proton transfer reactions, metal complex formations and the formation of enzyme-substrate complexes.
- AN.019.T64
Temperature calibration of T-Jump Instrument TJ-64 using Tris/Phenol Red
Keywords
- T-Jump
- TJ-64
- Temperature calibration
- Phenol red
- Tris
- AN.020.T64
Ionic Strength Dependence of the Heating Time in T-Jump Instrument using Fluorescence
Keywords
- T-Jump
- TJ-64
- N-Acetyl-L-tryptophanamide
- Fluorescence
- Heating time

